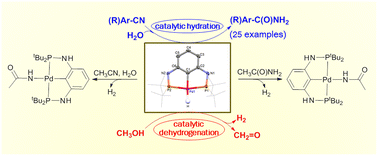
Reactions and catalytic applications of a PNCNP pincer palladium hydride complex - Dalton Transactions (RSC Publishing)

Nanometer-Size Effect on Hydrogen Sites in Palladium Lattice | Journal of the American Chemical Society
INSTABILITY IN A PALLADIUM HYDRIDE SYSTEM DUE TO A FAST ELECTRICAL PERTURBATION CAUSED BY A PULSED POWER SYSTEM

Electronic structure and crystal phase stability of palladium hydrides: Journal of Applied Physics: Vol 116, No 17
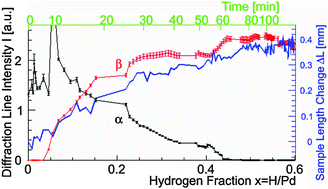
Change in the crystalline structure during the phase transition of the palladium–hydrogen system - Physical Chemistry Chemical Physics (RSC Publishing)

Nanometer-Size Effect on Hydrogen Sites in Palladium Lattice | Journal of the American Chemical Society

Superstoichiometric hydride PdHx ≤ 2 formed by electrochemical synthesis: Dissolution as molecular H2 proposed - ScienceDirect

Synthesis of Stable Shape-Controlled Catalytically Active β-Palladium Hydride | Journal of the American Chemical Society

Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal‐Organic Framework Coating Effects - Dekura - 2019 - ChemPhysChem - Wiley Online Library

Nanoporous Palladium Hydride for Electrocatalytic N2 Reduction under Ambient Conditions - Xu - 2020 - Angewandte Chemie International Edition - Wiley Online Library
Particle Size Effect of Hydride Formation and Surface Hydrogen Adsorption of Nanosized Palladium Catalysts: L3 Edge vs K Edge X-

Nanometer-Size Effect on Hydrogen Sites in Palladium Lattice | Journal of the American Chemical Society
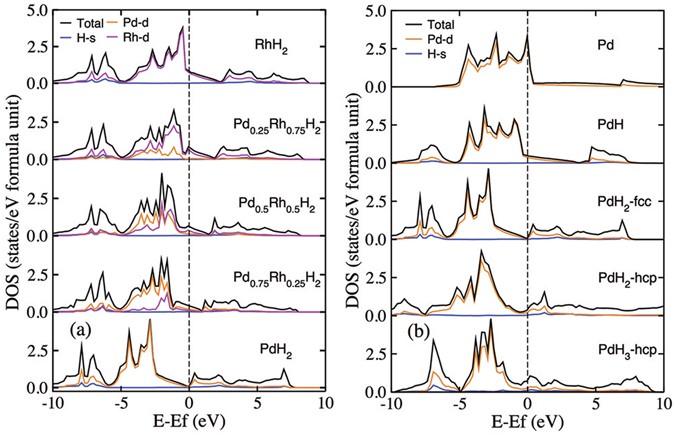
Formation and electronic properties of palladium hydrides and palladium-rhodium dihydride alloys under pressure | Scientific Reports
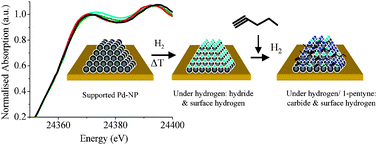
The irreversible formation of palladium carbide during hydrogenation of 1-pentyne over silica-supported palladium nanoparticles: in situ Pd K and L3 edge XAS - Physical Chemistry Chemical Physics (RSC Publishing)
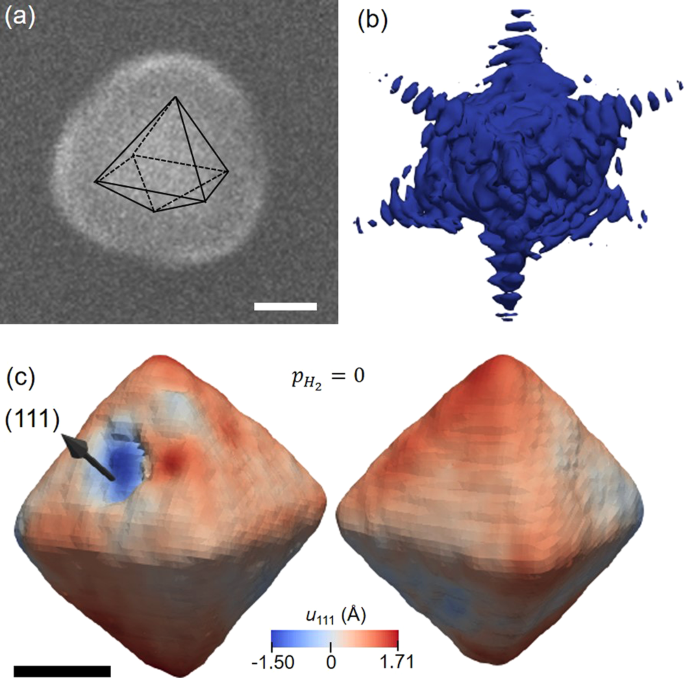
Structure of a seeded palladium nanoparticle and its dynamics during the hydride phase transformation | Communications Chemistry





